Czy ARMOR znajduje się w Wykazie Produktów Biobójczych?
Share
Jedno z najczęściej zadawanych pytań dotyczące produktów dezynfekujących, zwłaszcza w kontekście epidemii koronawirusa, dotyczy ich działania w zakresie eliminacji wirusów. Jak zatem można mieć pewność, że dany preparat jest skuteczny w tym zakresie?
Jedyne oficjalne źródło informacji
Nie zawsze możemy w stu procentach polegać na deklaracjach producentów, którzy niekiedy decydują się na wprowadzenie produktu na rynek bez uprzedniej jego rejestracji, co stanowczo nie powinno mieć miejsca. Jedynym oficjalnym źródłem, na którym możemy polegać i w którym odnotowane są produkty posiadające numer pozwolenia na obrót jest Wykaz Produktów Biobójczych. W bazie tej znajdziemy wszystkie oficjalnie dopuszczone do sprzedaży produkty biobójcze wykazujące działanie wirusobójcze do stosowania w miejscach użyteczności publicznej. Główny Inspektorat Sanitarny zachęca do systematycznego sprawdzania informacji zawartych w tym wykazie, by mieć pewność, że wybrany przez nas preparat w nim widnieje.
Wykaz ten jest dostępny pod adresem: http://bip.urpl.gov.pl/pl/biuletyny-i-wykazy/produkty-biob%C3%B3jcze
Jakie informacje znajdują się w Wykazie Produktów Biobójczych i czy są aktualne?
Jak podaje ustawa o produktach biobójczych, jeśli są one udostępniane i stosowane na terytorium Polski, wówczas podlegają wpisowi do Wykazu Produktów Biobójczych. Wykaz taki jest dostępny na stronie Urzędu Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych w Biuletynie Informacji Publicznej. Wykaz Produktów Biobójczych prowadzi Prezes Urzędu, który aktualizuje go nie rzadziej niż raz w miesiącu. Co ważne, baza ta obejmuje produkty biobójcze przeznaczone do dezynfekcji zarówno do użytku prywatnego jak i publicznego.
Jakie jeszcze informacje znajdziemy w Wykazie Produktów Biobójczych?
- nazwę produktu biobójczego
- imię i nazwisko oraz adres albo nazwę (firmę) oraz adres siedziby podmiotu odpowiedzialnego lub posiadacza pozwolenia albo zezwolenia na handel równoległy
- imię i nazwisko oraz adres albo nazwę (firmę) oraz adres siedziby wytwórcy produktu biobójczego
- nazwę chemiczną substancji czynnej lub substancji czynnych lub inną pozwalającą na ustalenie tożsamości substancji czynnej oraz, jeżeli są dostępne, jej numer WE i numer CAS, o których mowa w części I załącznika VI do rozporządzenia nr 1272/2008 oraz określenie jej zawartości w produkcie biobójczym w jednostkach metrycznych
- grupę produktową
- postać użytkową produktu biobójczego i jego przeznaczenie
- zakres i warunki obrotu produktem biobójczym lub opis jego zastosowania
- rodzaj opakowania
- treść oznakowania opakowania produktu biobójczego w języku polskim
- okres ważności produktu biobójczego
- numer pozwolenia, zezwolenia na handel równoległy albo pozwolenia na obrót oraz datę ich wydania i termin ważności
Źródło: http://www.urpl.gov.pl/pl/produkty-biob%C3%B3jcze/wykaz-produkt%C3%B3w-biob%C3%B3jczych
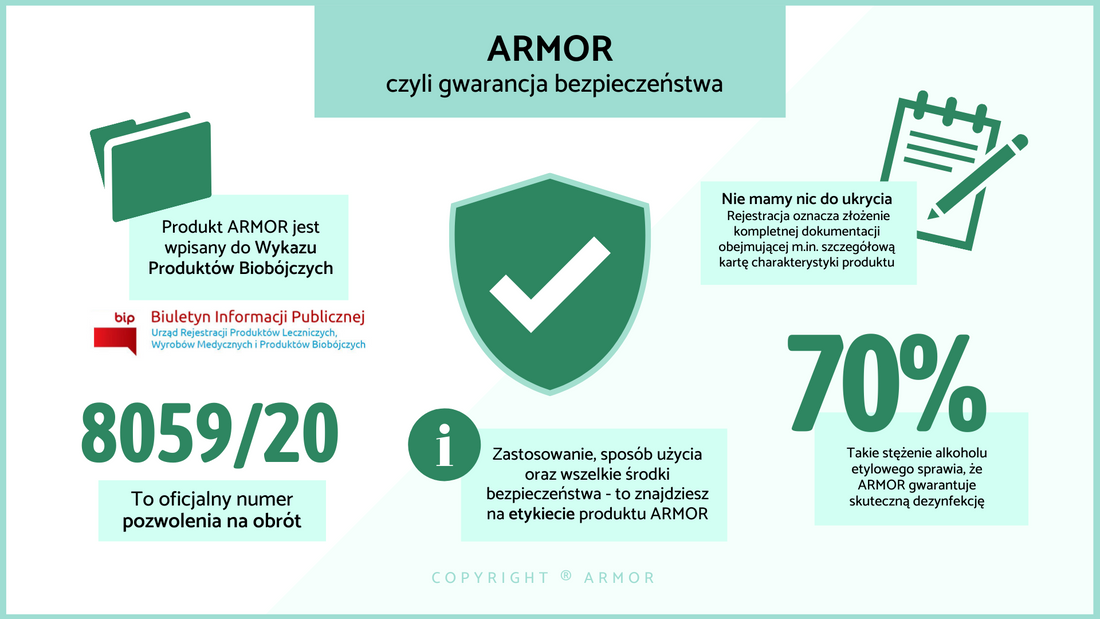
Czy ARMOR znajduje się w Wykazie Produktów Biobójczych?
Oczywiście! Nasz produkt jest oficjalnie zatwierdzonym produktem biobójczym o działaniu wirusobójczym, wprowadzonym do Wykazu Produktów Biobójczych jako ARMOR hand sanitizer z numerem pozwolenia na obrót 8059/20. Baza potwierdza również 70%-owe stężenie alkoholu etylowego, które jest gwarancją skutecznej dezynfekcji oraz podaje informacje na temat szerokiego zastosowania produktu, m.in. w gospodarstwach domowych, szkolnictwie oraz przemyśle. Wybierając ARMOR masz także gwarancję otrzymania produktu z etykietą, na której znajdziesz wszystkie wymagane informacje, takie jak sposób użycia lub postępowanie w przypadku dostania się preparatu do oczu. Z ARMOR'em możesz czuć się bezpiecznie.